
Lupi C, Pescetelli A (2008) Treatment of some liquid waste associated with lead battery recycling. īrandon NP, Pilone D, Kelsall GH, Yin Q (2003) Simultaneous recovery of Pb and PbO 2 from battery plant effluents. ĭai F, Huang H, Chen B et al (2019) Recovery of high purity lead from spent lead paste via direct electrolysis and process evaluation. Įllis TW, Mirza AH (2010) The refining of secondary lead for use in advanced lead-acid batteries. īallantyne AD, Hallett JP, Riley DJ et al (2018) Lead acid battery recycling for the twenty-first century. Li M, Liu J, Han W (2016) Recycling and management of waste lead-acid batteries: a mini-review. Zhang W, Yang J, Wu X et al (2016) A critical review on secondary lead recycling technology and its prospect. By using this anode, the specific energy consumption varied over the 0.04–0.17 kWh/kg range. Preliminary electrorefining tests highlighted that the best results were obtainable by using the anode cast in a titanium holder that allowed to obtain high-purity lead, high anode durability, and low quantity of anodic mud. Independently on the anode type, the complex composition of the anode requires to design a special electrolytic cell composed by two different compartments. Three different anode types were cast and tested. Several tests have been carried out in order to determine the starting mix composition that allows to reduce the process temperature and then SO 2 emissions. In this work, where only the anode preparation was a pyrometallurgical process, this problem has been overcome by limiting the process temperature. Many efforts have been devoted to solving this concern.
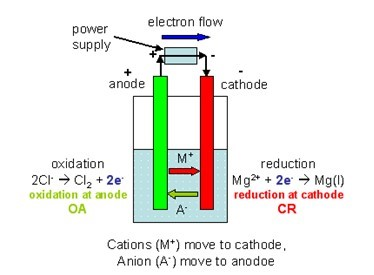

Lead acid batteries are processed mainly by using pyrometallurgical operations with problems related to SO 2 evolution.


 0 kommentar(er)
0 kommentar(er)
